Bovaer Is An Anti-Fertility Weapon
Citation censorship, cover-up, explicitly listed harms ignored, mandated and expansive
Bovaer is a drug product added to cows feed, which in turn is ending up in cows milk. The drug is made by DSM-Firmenich AG.
They claim it is for reducing methane produced by cows, by disrupting the methanogenesis (methane production) of bacteria in their gut by attacking enzyme methyl coenzyme M reductase (MCR), according to the United States Patent and Trademark Office (USPTO).
Bovaer contains 3-Nitrooxypropanol (3-NOP), silicon dioxide (sand), and Propylene Glycol. DSM-Firmenich AG claims it is “misinformation” to say this drug harms people or that they received financing from Bill Gates.
This is despite the fact the Gates Foundation has them listed on their website as receiving a grant from them for work in Malaria. Before rolling this out, DSM-Firmenich AG attended the Glasgow Climate Change Conference.
Drug Harms
The FDA (Food and Drug Administration) ironically lists 3-NOP (3-Nitrooxypropanol) as “not for human use” on their website (page 4), explicitly noting reproductive harms for males.
PubChem notes a fertility warning and specifies toxicity if swallowed.
Propaganda websites with financial conflicts of interest (per previous Daily Beagle reporting) have attempted to claim this is false, however studies — which they have attempted to censor and hide — reveal fertility harms.
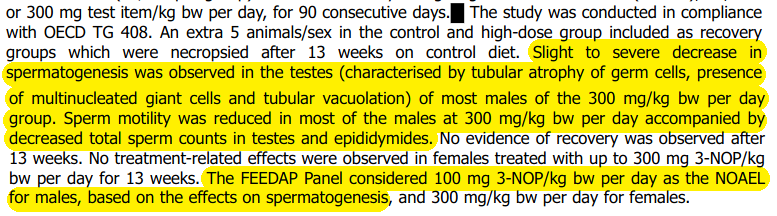
Studies confirm it causes harm to sperm in rats and beagles, causing “slight to severe” damage, a “severe reduction” in spermatogenesis. That is the process by which sperm cells mature.
The study lists additional harms, including alarming impacts on the testes. However, rather than using this as evidence the product must not be given to animals or humans, they simply opted to lower the dosage, rather than addressing or reporting upon the harms discovered.
This is despite the fact FEEDAP (EFSA Panel on Additives and Products or Substances used in Animal Feed) could not conclude on the margin of safety of the material, despite rubber stamping it as safe in the face of contrary evidence.
The same study found 3-NOP is stable in the stomach of rats…
Data from both single dose studies showed that 3-NOP orally administered to rats are stable in the stomach
…it was also in high concentrations…
in the liver (184.5 lg 3-NOP-eq/g), adrenal glands (159.3 lg 3-NOP-eq/g) and kidney (118.4 lg 3-NOP-eq/g).
All locations it shouldn’t be in. 3-NOP is converted into NOPA (nitroxypropionic acid) and 3-HPA (3-Hydroxypropionic acid), which was found in the testes of the rats.
In the epididymides and testes, 3-HPA and NOPA were present being 3-HPA the major compound, from 60 to 84% TRR in epididymis and testes, respectively. In all these organs, some other compounds were detected but not identified.[sic]
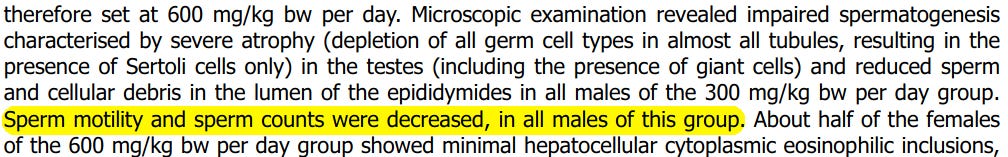
The study goes on to explicitly state that “Sperm motility and sperm counts were decreased, in all males of this group”.
Going along with the dystopian theming, there are unprecedented levels of censorship in this peer-reviewed article claiming safety. 42 of the 88 citations censored, or 47.7%, with a blanket statement with circular reasoning that it was censored because a request to censor it was issued.
Relevant information or parts of this scientific output have been blackened in accordance with the confidentiality requests formulated by the applicant pending a decision thereon by the European Commission.
FEEDAP falsely bases their claim for safety on a paper where nearly 50% of the citations have been censored and are therefore unverifiable. It is evident then this claim of safety is a scam, especially in light of the evidence of harms.
This is what 42 censored citations looks like:
They even censored the citation numbers in the paper:
This is a major red flag this drug is not safe and that there is an attempt to poison the general public by sweeping blatant concerns of toxicity under the carpet. It is clearly an anti-fertility weapon, and their only excuse will be hair-splits on dosage amounts when it’s not even remotely required to be given to cows.
Extent
How far do they intend to adopt this poison? Very far. The UK Government are mandating Bovaer for all cows, and they already have a production facility in Dalry, Scotland, which also produces food for humans. That’s right, the same location using the anti-fertility drug for cows is also producing food for humans.
Previously in 2022, DSM took over Firmenich (DSM-Fimenich AG is a merger between two companies). Firmenich are a flavour and fragrance firm, and they’re looking to also introduce “foods” into the “alternative foods and nutrition” market, aiming at vegan and “alternative” foods, so even if you try to avoid meat they’re still going to be poisoning the food supply. This is a war on humanity.
Arla foods, a Danish-Swedish company, are adopting DSM-Firmenich’s Bovaer, and are acting as the company’s front runner. Arla own brands such as Lurpak, Anchor, and Apetina. They’re working with Aldi, Asda and Morrisons (Asda have a 4-year partnership with Arla), Tescos and Müller, they supply Lidl’s milk.
Sainbury’s and Waitrose both previously (in 2023) dropped Arla, but unfortunately replaced it with Müller, whom has a working partnership with Arla. Arla also have unique exclusive products with store brand Iceland. That said, it was last reported Medina (in 2017) supplies Iceland’s milk, it is not clear if this has since changed.
Marks&Spencer (M&S) did not explicitly state any ties with Arla, however they have explicitly stated they are committed to supposedly reducing emissions from cows, so it is very likely they are doing the same thing.
Arla are also working with Dale Farm in Northern Ireland, and APC Microbiome Ireland looking at infant formula. So not even the children will be safe.
Arla acquired Yeo Valley back in 2018, so all Yeo Valley milk is also affected. Arla have also partnered with Starbucks. Starbucks also claim to be working directly with farmers to “lower emissions”.
Arla are also working with McDonalds. Arla are also being assisted by Burges Salmon and HSBC. Arla, along with classically evil Nestle, are donating their toxic milk crap to food banks, ensuring the poor also get access to this anti-fertility weapon.
Arla are expanding to Belgium, DSM-Firmenich are expanding to Brazil and Chile, and Arla have operations in the USA, including selling Castello cheese.
The Government Will Not Protect You
It is evident the government and corporations are waging a war on humanity with this blatant disregard for evidence indicating harms. There’s a famous line that is currently going around online, that goes…
…you are the carbon they want to reduce.
This time it is war. They couldn’t get you to accept the shots, so now they’re poisoning the food and drinks supply. This isn’t their first poisoning attempt, or their second attempt, or even their third, or fourth. It will be impossible to remain passive in the face of your food supply being contaminated to this extent. It won’t just stop with the UK; they will want this anti-fertility drug worldwide.
Humanity must act.
Related Articles To Read
Near To Zero: Sterilisation Viruses and "Malnutrition Vaccines"
Scotland Conspires To Deny Unvaccinated Fertility Treatments
Found this informative?
Help inform?
Thoughts, dear reader?

















Another must see is this science memo from New Zealand (2022).
The regulators put Bovaer as GHS Category 2 for reproductive toxicity.
(It's worse still for rats).
***
... The EPA considers that the mechanism of toxicity is not sufficiently characterised to enable a firm
conclusion that the toxicity to the testis and epididymis is a rat specific finding but does suggest that
other species may be less sensitive to the compound. Therefore, the EPA does not consider that it
can be concluded that this finding has no human relevance. Consequently, the clear evidence of an
adverse effect on reproduction in a combined toxicity screening study in the absence of other toxic
effects, supported by evidence from repeat dose studies showing the male reproductive organs to be
the target organs for toxicity justifies classification for reproductive toxicity. The EPA concludes that
the appropriate classification is GHS Category 2 for reproductive toxicity.
https://www.google.co.uk/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.epa.govt.nz/assets/FileAPI/hsno-ar/APP204100/APP204100-Science-Memo-Final.pdf&ved=2ahUKEwi13dyMhLeKAxWYQUEAHVixJw4QFnoECBcQAQ&usg=AOvVaw3vrxcW6687mcstvafc9aHm
The "N" in 3-nitrooxypropanol got me thinking about metabolites, such as N-nitrosamines, which have loooong been associated with cancer. It's why we were told not to eat too much bacon, etc.
***
Induction of liver tumors in Wistar rats by sodium nitrite given in pellet diet
M Aoyagi et al. J Natl Cancer Inst. 1980 Aug.
https://pubmed.ncbi.nlm.nih.gov/6931259/
***
Which is why nitrite formation as a breakdown metabolite of Bovaer presents "a bit of a problem".
The fix is simple. Redefine nitrites as safe, and no longer linked to cancer 🤞👌🤫
***
Nitrites are now safe again
... An oxidant called 3-nitrooxypropanol (3-NOP, aka Bovaer) binds to the active site of MCR and oxidizes an essential SH group, inhibiting production of methane in bacteria. The effect only lasts for 5 hours at a concentration of 1 μM,[7] after which the bacteria reactivate the enzyme. 3-NOP is rapidly absorbed by the intestine and makes its way through the animal's bloodstream, and is then denitrated in the liver. This probably explains why 100× more 3-NOP is needed to produce the effect in the animal than in cultured bacteria. 3-NOP is metabolized to nitrate, nitrite, and 1,3-propanediol.
https://randombio.com/nitrite.html